Technological solutions
Initial process sizing. Basic Pharma Engineering.
Initial process sizing. Basic Pharma Engineering.

And then is necessary to start at some point. Because pharmaceutical plants are involving numerous interrelated processes.
Plant engineers split the design in phases: Basic engineering, Detailed Engenering and finally the Executive Engineering.
From Lyoptimus we can be collaborating at early stages of the plant definition, providing key estimates for the best decision making.
Building the User Requirement Specifications
Initial process sizing. Basic Pharma Engineering.

When is time for the acquisition of large scale freeze dryers is absolutely necessary to accurately detail the User Requirement Specifications.
Nevertheless, it should be avoided to over specify the units, by including all the options and top notch features from the different manufacturers. On the other hand, the key features for your application should not be missing.
Getting support from a freeze drying expert will speed up and ensure this important step.
Detailed sizing. Layout, consumptions and components
Industrial freeze dryers are modular units that allow customization, specially regarding the distribution of the main (more voluminous) subassemblies: condenser, vacuum, refrigeration and CIP bench, also the control cabinet.
Even if there is a good consensus about the sizing of the different subsystems, each application may require an adjusted design.
Choosing the proper components from reputed brands, can make the difference, increasing the reliability of the lyophilizer, and the homogeneity on the production batches.
Design Qualification (DQ). Functional, Hardware and Software Specifications (FS-HDS/ SDS)
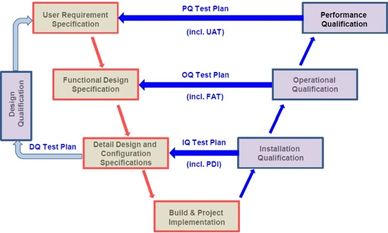
Just after ordering a lyophilizer starts the design phase. As per the Good Manufacturing Practices (GMP), the freeze dryer needs to be designed in detail prior the acquisition of components, the metal workshop and the assembling works.
The Design Qualification assures that the URS will be fulfilled by the dessing proposed, hardware and software, for the required funtionality.
A well documented Detailed Design is the best guarantee of a clear understanding between the freeze dryer manufacturer and the freeze dryer user, for a smooth and efficient project development.
From Lyoptimus we can join the working team for improving the detailed design and related documentation.
Factory & Site acceptance (FAT/SAT). Installation, Operational & Process Qualification (IQ/OQ-PQ)
Factory & Site acceptance (FAT/SAT). Installation, Operational & Process Qualification (IQ/OQ-PQ)

After long months for designing and manufacturing the freeze dryer, it is time to test the operation of the unit.
First at the manufacturer factory (FAT), then again at the final user plant (SAT), finally for the qualification, to allow starting the pharmaceutical production (IQ/OQ-PQ).
The number of documents to review and approve is large, also are numerous the tests and checks to be performed to all the subsystems.
It is necessary implement a good strategy combined with the proper knowledge and expertise, for making a success the last steps of the process, and Lyoptimus can be by your side for it.
Contact Us
From Barcelona
We are based in Barcelona and we operate internationally
Subscribe
Periodically we are pubishing our works: topics of interest, white papers and scientific papers. Take a look to our publications section. When subscribed to our site, you will not be missing any of our new releases.
